Only IZERVAY directly targets C5, preserving upstream homeostasis of the complement system7,18
Designed differently19
SYNTHETICALLY DEVELOPED
RNA aptamer technology
Low immunogenicity
Low likelihood of inducing an immune response.
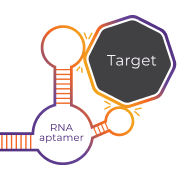
High binding affinity
Strong and specific inhibition of the target.
Targets differently7,20

By inhibiting C5, IZERVAY may:
- Reduce inflammation
- Reduce retinal cell death
- Reduce loss of photoreceptors
Comprehensive packaging for seamless administration7
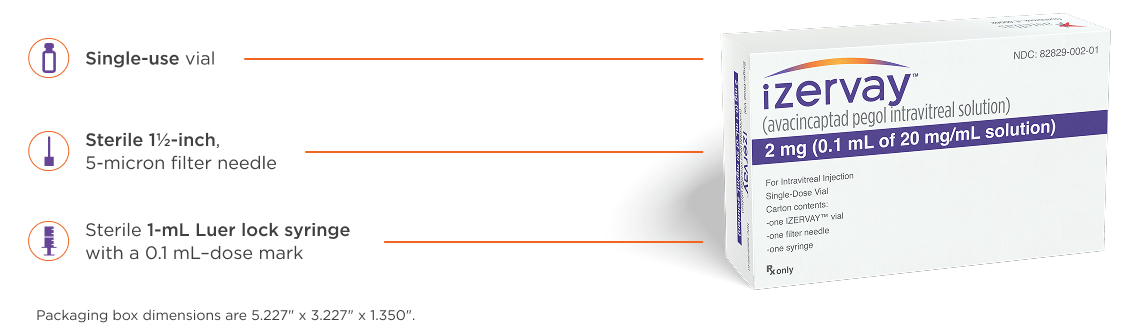

Preparation and administration

IZERVAY can be administered with a 30-gauge injection needle, which may decrease patient discomfort.7,21

For added convenience in your practice, unopened vials of IZERVAY can remain unrefrigerated for up to 24 hours.7
RNA=ribonucleic acid.